Building structures made of marble and limestone are mostly affected by acid rain as the acid eats the calcium compounds in the structures.
The effect of acid rain on a marble structure lab.
Acid rain acid rain effects on human made structures.
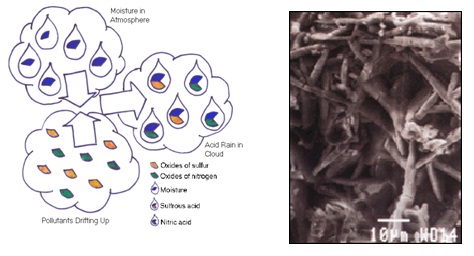
Acid rain is referring to rain with a ph4 or lower.
However the main effect is man made burning of the coal.
Higher the concentration of hydrochloric acid the faster the reaction will take place because there will be more hydrochloric acid particles to collide with the marble chip particles therefore resulting in a quicker reaction.
Acid deposition also affects human made structures.
In exposed areas of buildings and statues we see roughened surfaces removal of material and loss of carved details.
Objectives the objectives in this investigation are 1 to demonstrate and measure the effect of acid rain on exposed stone surfaces and 2 to calculate the rate of acid degradation of limestone.
They let nitric acid into the atmosphere.
The most notable effects occur on marble and limestone which are common building materials found in many historic structures monuments and gravestones.
Also in the structure of the carbonate ion are any of the oxygens bonded to one another or all the oxygens bonded to the carbon atom.
As a result it has led to weathering of buildings corrosion of metals and peeling of paints on surfaces.
Over time statues made of marble will suffer ill effects from exposure to acid rain.
Acid rain has corrosive effects because it eats into metals and stone.
It is well established that either wet or dry deposition of sulfur dioxide significantly increases the rate of corrosion on limestone sandstone and marble.
This lets of sulfuric oxides the other one is cars trucks etc.
When sulfurous sulfuric and nitric acids in polluted air and rain react with the calcite in marble and limestone the calcite dissolves.
A combination of natural events such as volcanic.
The effects of acid rain on building material eg.
The lower the concentration the weaker the reaction will be as there will be fewer particles so less chance of.
Based on the information described above about the calcium ion and the formula of calcium carbonate caco 3 deduce the charge of the carbonate ion.
Environmental protection agency epa has defined acid rain as a term that refers to a mixture of dry and wet material containing higher than normal amounts of sulfuric and nitric acids that deposits out of the atmosphere.
Questions on effects of acid rain.
Natural causes are lightning strikes volcano eruptions and also dead and decaying foods.
Acid rain experiment with marble chips and hydrolic acid.
Stone surface material may be lost all over or only in spots that are more reactive.